Abstract
Introduction: The burden of beta-thalassemia (β-thal) is well documented but, to our knowledge, there are no systematic literature reviews (SLRs) on the burden of alpha-thalassemia (α-thal). To fill this gap, we conducted an SLR to characterize the clinical (complications, treatment patterns, or mortality), health-related quality of life (HRQoL), and economic burden associated with α-thal and report on evidence gaps.
Methods: An SLR was conducted for publications on clinical burden, HRQoL, HCRU/costs and economic evaluations in thalassemia. MEDLINE, Embase, EconLit and Evidence-Based Medicine Reviews and relevant conference websites (2019-2021) were searched. Real-world, observational studies and economic evaluations were selected that reported on α-thal and any outcome data of interest; for clinical burden, only studies in adults (≥18 years) were included.
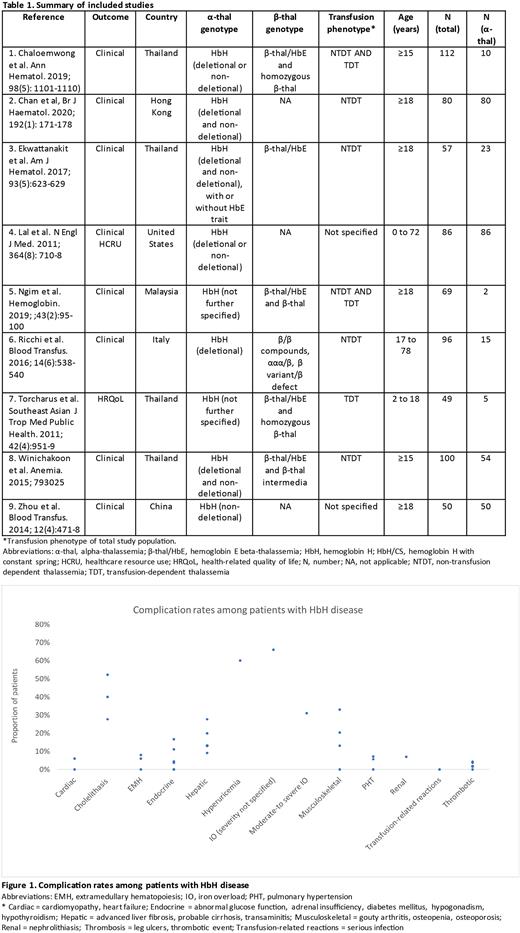
Results: Across 7,881 search hits in thalassemia, 9 studies reported relevant data on α-thal (see Table 1).1-9 No evidence was identified on economic evaluations. Only three studies were exclusively in α-thal patients (sample size 50-86) and were limited to deletional (DEL) HbH and/or non-deletional (non-DEL) HbH, including Constant Spring (CS).2, 4, 9 These studies found that among adults with DEL and/or non-DEL HbH, almost 1 in 3 had moderate-to-severe iron overload (31%),2 and 2 out of 3 had iron overload of unspecified severity (66%).9 Advanced liver fibrosis was present among 1 in 5 patients (20%); 9% with probable cirrhosis.2 Complications including moderate-to-severe liver iron overload (53% vs. 25%, P=0.03) and median liver iron concentration (median [range]: 7.8 [1.7-17.7] vs. 2.9 [0.1-15.0] mg/g dry weight, P=0.002) were significantly higher in non-DEL versus DEL HbH adult patients with non-transfusion dependent thalassemia (NTDT).2 In addition, among HbH/CS transfusion-dependent thalassemia (TDT) and NTDT adult patients, iron overload was further exacerbated among those who were splenectomized compared to those who were not (80% vs. 52%, P=0.037).9 The other studies reporting clinical data were from a mixed (α-thal and β-thal) population where data for α-thal was reported separately (6 studies with sample sizes ranging from 2 to 54 with α-thal).1, 3, 5-8 Across these, the data also demonstrated a high burden of clinical complications for HbH and/or HbH/CS including iron overload (52.2%, 1 study)3, cholelithiasis (27.7% to 52.2%, 3 studies3, 6, 8), abnormal liver function (27.7%, 1 study8), osteoporosis (0.0% to 20.4%, 3 studies3, 6, 8) and hyperuricemia (60.0%, 1 study).1 Complication rates among α-thal patients across studies can be seen in Figure 1. With regard to treatment patterns, among patients with DEL and/or non-DEL HbH, the majority had historical/occasional transfusion (46.7% to 87.0%, 3 studies)2, 3, 6, followed by iron chelation therapy (6.0% to 52.2%, 5 studies)2, 3, 6, 8, 9 and splenectomy (10.0% to 14.8%, 3 studies).2, 6, 8 A single study on HRQoL in TD HbH and β-thal (age range: 2 to 18 years) found that HRQoL total score, and subdomains, psychological, emotional, social, and school functioning (as measured by Pediatric Quality of Life Inventory) were similar between HbH and β-thal patients, except for physical functioning.7 For physical functioning, homozygous β-thal patients had the best HRQoL, followed by HbH patients, with β-thal/Hb E patients demonstrating the poorest HRQoL (mean [SD]: 76.52 [15.23] vs. 61.16 [18.39] vs. 56.88 [17.17], respectively, P=0.008).7 This study was limited by a small sample size (n=49) with only 5 HbH patients.7 Lastly, one study on HCRU among adult and pediatric patients with DEL HbH and HbH/CS, found that patients with HbH/CS had a significantly increased number annual clinic visits, by a factor of 1.7, and hospital admissions, by a factor of 3.9, versus those with HbH (P<0.001).4
Conclusions: To our knowledge, this was the first SLR investigating the clinical, HRQoL and economic burden of α-thal. Most of the evidence was in HbH and HbH/CS adult patients, and complications, with no data on economic evaluations, and limited data on HRQoL and HCRU/costs. Our SLR highlights the need for further research to fully characterize the burden of this disease. Where reported, DEL and/or non-DEL HbH adult patients experience clinical complications across a range of conditions and generally, children and adolescents with HbH experience similar HRQoL burden as those with β-thal.
Disclosures
Musallam:Celgene Corp (Bristol Myers Squibb): Consultancy; Agios Pharmaceuticals: Consultancy; Novartis: Consultancy; CRISPR Therapeutics: Consultancy; Vifor Pharma: Consultancy; Pharmacosmos: Consultancy. Lombard:Agios Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Gilroy:Agios Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Vinals:Cytel Inc.: Current Employment; Evidera: Ended employment in the past 24 months. Tam:Cytel Inc.: Current Employment; IQVIA: Ended employment in the past 24 months. Rizzo:Cytel Inc.: Current Employment, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal